A Pilot Study
Glutathione
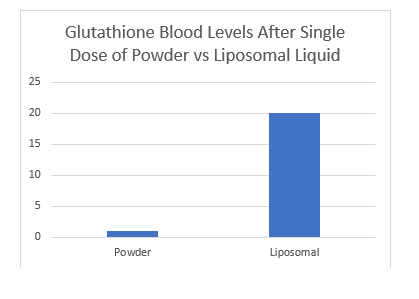
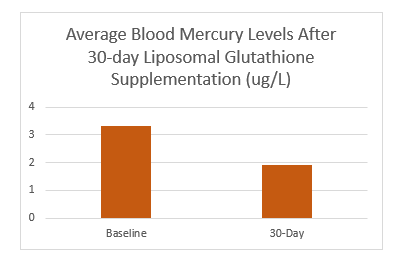
A preliminary clinical trial was conducted to evaluate the impact of liposomal glutathione, a powerful tripeptide, on blood glutathione levels two hours post-administration (N=4). Additionally, seven healthy participants were given 750 mg of liposomal glutathione (CELLg8®) twice daily for 30 days. The study demonstrated a reduction in bilirubin and a 39% decrease in blood mercury, indicating enhanced detoxification and improved liver function. No other significant changes were noted in the 21 blood panel tests, except for an improvement in kidney and liver function. Further testing is underway to expand the sample size to 12, aiming to provide more robust data on the benefits of this liposomal peptide formulation.


A Pilot Study
Absorption, Safety, and Detoxification Effects of Oral Liposomal Glutathione
Abstract
Abstract
A pilot clinical study was undertaken to assess the effect of liposomal glutathione, a vital tripeptide, on blood levels and detoxification processes (N=2). Peak results showed that seven additional healthy participants who took 750 mg of liposomal glutathione (CELLg8®) twice daily for 30 days experienced improved detoxification and liver function, evidenced by reduced bilirubin and a 39% decrease in blood mercury levels. All other indicators in the 21 blood panel remained healthy during the 30-day period, without significant changes. Further testing is currently underway to expand the study to an N of 12, aiming to strengthen the data on this liposomal peptide formulation.

A pilot clinical study was undertaken to assess the effect of liposomal glutathione, a vital tripeptide, on blood levels and detoxification processes (N=2). Peak results showed that seven additional healthy participants who took 750 mg of liposomal glutathione (CELLg8®) twice daily for 30 days experienced improved detoxification and liver function, evidenced by reduced bilirubin and a 39% decrease in blood mercury levels. All other indicators in the 21 blood panel remained healthy during the 30-day period, without significant changes. Further testing is currently underway to expand the study to an N of 12, aiming to strengthen the data on this liposomal peptide formulation.